The decision is yours.
COVID-19 vaccines are safe, effective, and recommended for everyone 6 months and older.
For more information
- Ask us a question about COVID-19 and vaccines
- Wisconsin Department of Health Services COVID-19 vaccine resources
- View our videos about the COVID-19 vaccines – English & Spanish
Questions about COVID-19 vaccines? Get information to make a decision that’s right for you. | ¿Tiene preguntas acerca de las vacunas contra COVID-19?
Vaccine Booster Doses
What is a booster dose of the vaccine?
Booster doses are common for many vaccines. A “booster dose” is a supplemental vaccine dose given to people when a person has completed their vaccine series and protection against the virus has decreased over time. The booster dose is intended to boost your immune system for better, long-lasting protection. Safety data from other countries and the Centers for Disease Control and Prevention (CDC) shows that booster and additional doses are safe.
What are the benefits to getting a booster dose?
A booster dose provides optimal protection from a COVID-19 infection. Booster doses reduce the risk of serious illness. During the delta and omicron waves, adults who received a booster dose were less likely to be infected, hospitalized, or die from COVID-19. Unvaccinated adults had nearly 5 times higher risk of infection than adults who were fully vaccinated with a booster.
Who should get a booster dose?
Everyone ages 5 years and older should now get a booster shot.
DHS recommends that the following people get a booster dose if they received a:
- Johnson & Johnson COVID-19 vaccine at least 2 months ago
- Moderna primary vaccine series at least 5 months ago and are 18 years and older
- Pfizer-BioNTech primary vaccine series at least 5 months ago and are 12 years and older
The CDC recommends people receive an mRNA COVID-19 vaccine booster (like Pfizer or Moderna) over Johnson & Johnson’s COVID-19 vaccine in most situations. For more information about booster doses, visit: https://www.dhs.wisconsin.gov/covid-19/vaccine-dose.htm
Who has the option to get a second booster dose?
A second booster dose can help increase protection for higher-risk individuals. A second booster dose of the Pfizer-BioNTech or Moderna vaccine may be administered at least 4 months after the first booster dose to people who are:
- 50 years of age and older
- 12 years of age and older who are moderately to severely immunocompromised
- 18 years of age and older and received both a first dose and booster dose of the Johnson & Johnson vaccine
Who should get an additional vaccine dose?
An additional dose is recommended for people who may not have received adequate protection from their initial vaccine series. People ages 5 years and older with moderately to severely weakened immune systems should get an additional dose of vaccine 28 days after their 2nd shot.
For more information about additional doses, visit: https://www.dhs.wisconsin.gov/covid-19/vaccine-dose.htm
Kids and COVID-19
Is the COVID-19 vaccine available for children?
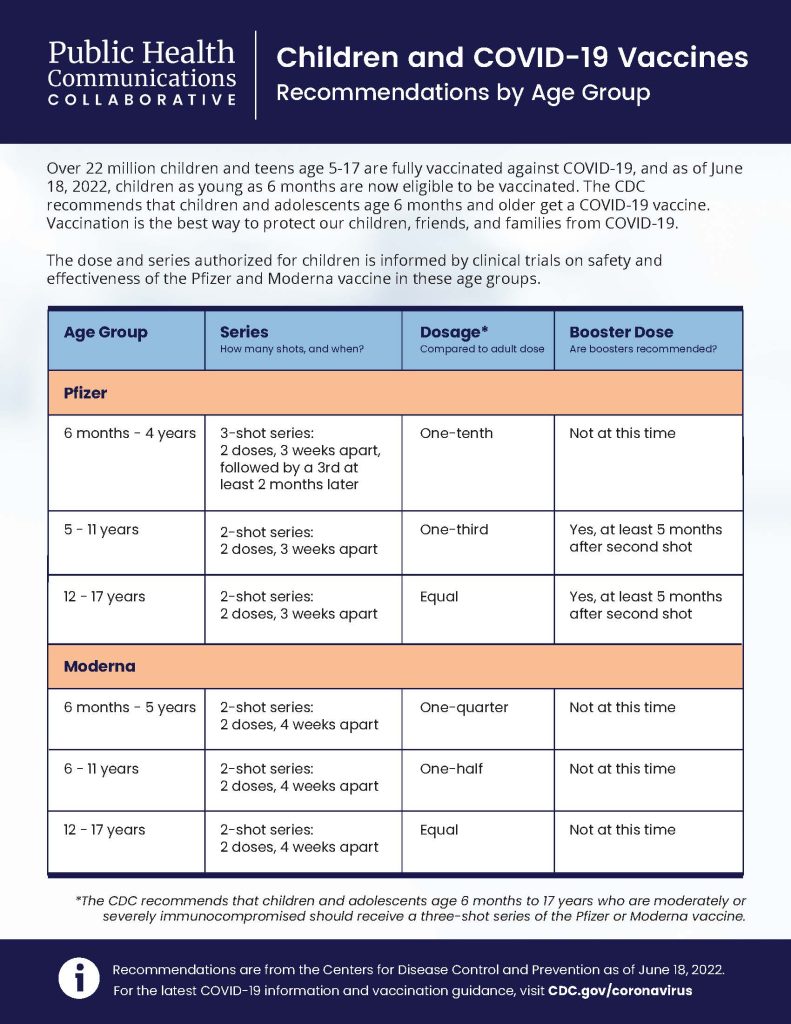
Children 6 months and older are now eligible to receive vaccination against COVID-19. The CDC recommends children be vaccinated as soon as possible. For more information on vaccines for children and teens visit:
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/children-teens.html
Why should my child get the COVID-19 vaccine?
COVID-19 has become one of the top 10 causes of pediatric death, and tens of thousands of children and teens have been hospitalized with COVID-19. While children and adolescents are typically at lower risk than adults of becoming severely ill or hospitalized from COVID-19, it is still possible. Medical experts are also learning about the long-term effects of COVID-19 in children. Getting your child vaccinated helps to protect your child, your family, and your community. Find more COVID-19 resources for parents and guardians (https://www.dhs.wisconsin.gov/covid-19/parents.htm).
Does getting vaccinated protect children and adolescents from serious illness and hospitalizations?
Yes. Children and adolescents are typically at lower risk than adults of becoming severely ill or hospitalized from COVID-19. But it’s still possible. More children were hospitalized from COVID-19 during the recent surge than at any point during the pandemic. Most children who were hospitalized were unvaccinated. For example, recent data showed that unvaccinated 12-17 year olds were 10 times more likely to be hospitalized with COVID-19 than vaccinated adolescents.
Is the COVID-19 vaccine safe for children?
Yes. Studies show that COVID-19 vaccines are safe and effective. Before being authorized for children, scientists and medical experts completed their review of safety and effectiveness data from clinical trials of thousands of children. By getting vaccinated, your child will be protected from getting sick and reduce the chances of spreading the virus to others. Like adults, children may have some side effects after COVID-19 vaccination. These side effects may affect their ability to do daily activities, but they should go away in a few days.
Vaccine Safety
How do we know COVID-19 vaccines are safe?
These vaccines have undergone the most intensive safety monitoring in U.S. history. Vaccines are authorized by the U.S. Food and Drug Administration (FDA), which sets strict standards for clinical trials. The FDA continues to monitor vaccines very closely for safety.
How are vaccines tested for safety?
Every vaccine must go through rigorous testing and inspection to ensure it is safe. Vaccines for COVID-19 followed a 3-phase process where there are several stages required before FDA authorization. After a vaccine is authorized by the FDA and made available to the public, FDA continues to monitor its safety very closely.
How were the vaccines made so quickly?
The science behind the breakthrough had a head start. Researchers had already made progress developing vaccines for other types of coronaviruses. The rapid spread of COVID-19 made developing these vaccines an international priority, unlocking billions of dollars in funding to ensure safety while moving with urgency to save lives.
What is the risk of having an adverse reaction to the vaccine?
An adverse reaction is an undesirable side effect that occurs after a vaccination. Side effects to the COVID-19 vaccines are typically mild and go away in one to two days. They include soreness in the arm, fatigue, and headaches. The risk of having a serious adverse reaction is very low. It is far lower than the risk of severe illness or death from contracting COVID-19.
Should I worry about long term side effects?
Serious side effects that would cause a long-term health problem are extremely unlikely. There has never been a ‘long-term’ vaccine side effect that emerged more than a few months after administration of a vaccine. Serious side effects that would cause a long-term health problem are extremely unlikely.
Do vaccines impact fertility?
There is currently no evidence that any vaccines, including COVID-19 vaccines, cause fertility problems. If you are trying to become pregnant now or want to get pregnant in the future, you may receive a COVID-19 vaccine.
Vaccine Efficacy
How do the COVID-19 vaccines protect me?
When we get a vaccine, it activates our immune response. The vaccine helps our bodies learn to fight off the virus. COVID-19 vaccines provide significant protection against serious illness and hospitalization.
I know lots of people who are vaccinated and boosted and they got COVID-19 anyway – do the vaccines even work?
Yes, the vaccines work. While it is true that many vaccinated people did get COVID-19, the vaccines continued to be highly effective at preventing severe illness and death. As of December 2021, people in Wisconsin who were not fully vaccinated for COVID-19 were 3 times more likely to be diagnosed with COVID-19, 10 times more likely to be hospitalized with COVID-19, and 14 times more likely to die from COVID-19 than people who were fully vaccinated.
Do vaccinations still help if I had COVID-19?
Yes. Getting vaccinated provides additional protection from future COVID-19 illness. Unvaccinated people who already had COVID-19 are more than twice as likely than fully vaccinated people to get COVID-19 again. Immunity from a prior infection combined with vaccination and receiving a booster provide strong protection from severe illness and death.
Do the vaccines protect against the variants?
So far, studies suggest that all vaccines authorized for use in the United States are effective in reducing risk of severe disease, hospitalization, and death against known variants. The latest data show that booster doses significantly increase protection from the Omicron variant.
Myocarditis and Multisystem Inflammatory Syndrome (MIS-C)
Can a COVID-19 vaccine cause myocarditis?
Myocarditis is the inflammation of the heart muscle. Symptoms include chest pain, shortness of breath, and rapid heartbeats. Myocarditis cases from vaccination are very rare and are typically mild if they do occur.
In fact, you are more likely to get myocarditis from getting sick with COVID-19 than from getting vaccinated. People who are infected with COVID-19 have 16 times the risk of myocarditis compared to those who are vaccinated. Myocarditis from COVID-19 illness is more severe.
The known risks of COVID-19 illness and possible long-term health implications outweigh the risk of COVID-19 vaccines.
What is multisystem inflammatory syndrome (MIS-C), and how can I protect my child from it?
Multisystem inflammatory syndrome (MIS-C) is a rare and dangerous complication of COVID-19 in children. Different body parts become inflamed, including the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal organs. MIS-C is caused by an immune reaction to the virus. Symptoms include rash, high fever, abdominal pain, high fever, vomiting, and diarrhea. As of February 18th, there have been a total of 183 MIS-C cases reported in Wisconsin since the beginning of the COVID-19 pandemic.
A CDC study was published on January 14th, 2022 examining Pfizer vaccine’s effectiveness in children ages 12 – 18 years. Researchers found Pfizer’s vaccine was highly effective at preventing MIS-C. Two doses of the Pfizer vaccine led to a 91% reduction in the likelihood of MIS-C. 95% of those who were hospitalized with MIS-C, and 100% of critically ill patients were unvaccinated. 39% of unvaccinated MIS-C patients required life support, but no fully vaccinated patients with MIS-C required life support.
Vaccine Access
How much does it cost?
There is no cost to getting vaccinated against COVID-19. COVID-19 vaccines are provided at 100% no cost to recipients. The federal government is providing the vaccine free of charge to all people living in the United States, regardless of their immigration or health insurance status.
What type of vaccine will I get?
Currently, three types of vaccines are authorized for use in the United States. Which vaccine you receive will depend on your age.
- Adults ages 18 years and older: Pfizer-BioNTech, Moderna or Johnson & Johnson
- Children and teens ages 12 to 17 years: Pfizer-BioNTech or Moderna
- Children ages 6 months to 11 years: Pfizer-BioNTech or Moderna
Every vaccine that is recommended by FDA and CDC has been thoroughly tested and found to be effective and safe.
Learn more at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html.
How old do I need to be to get vaccinated?
In the United States, everyone aged 6 months and older is currently eligible to receive COVID-19 vaccines.
Flu Vaccine and COVID-19
Do I need a flu vaccine if I wear a mask and practice physical distancing?
Yes. Wearing a mask and physical distancing can help protect you and others from respiratory viruses, like flu and the virus that causes COVID-19. However, the best way to reduce your risk of flu illness and its potentially serious complications is for everyone 6 months and older to get a flu vaccine each year. By getting a flu vaccine, you may also be protecting people around you who are more vulnerable to serious flu complications.
Can I get a flu vaccine and COVID-19 vaccine at the same time?
Yes, you can get a COVID-19 vaccine and a flu vaccine at the same time.
Even though both vaccines can be given at the same visit, people should follow the recommended schedule for either vaccine: If you haven’t gotten your currently recommended doses of COVID-19 vaccine, get a COVID-19 vaccine as soon as you can, and ideally get a flu vaccine by the end of October.
What is the difference between the flu and COVID-19?
Flu and COVID-19 are both contagious respiratory illnesses, but they are caused by different viruses. COVID-19 is caused by infection with a coronavirus (called SARS-CoV-2) and seasonal flu (most often just called “flu”) is caused by infection with one of many influenza viruses that spread annually among people.
Because some symptoms of flu and COVID-19 are similar, people may need to be tested to tell what virus is causing their illness. People can be infected with both a flu virus and the virus that causes COVID-19 at the same time. In general, COVID-19 seems to spread more easily than flu and causes more serious illnesses in some people. Compared with people who have flu infections, people who have COVID-19 can take longer to show symptoms and be contagious for longer. This FAQ page compares COVID-19 and flu, given the best available information to date.
Because symptoms of flu and COVID-19 are similar, how will I know if I have the flu or COVID-19?
Your health care professional may order a test to help confirm whether you have flu or COVID-19 or some other illness. Get more information on COVID-19 and flu testing and symptoms of COVID-19 and flu.
Novavax
What is the Novavax vaccine and is it available in the United States?
On July 19, 2022, the CDC updated its COVID-19 vaccine recommendations, approving the Novavax vaccine for emergency use authorization for adults 18 years and older. Novavax is a two-dose, protein-based COVID-19 vaccine that is currently being used in more than 40 countries and has been authorized by the European Union and the World Health Organization.
Novavax will now be the fourth COVID-19 vaccine available in the U.S., in addition to Pfizer, Moderna, and Johnson & Johnson. As a protein-based vaccine, Novavax is another option for people who are allergic to one of the components in a mRNA or viral-vector vaccine. The vaccine is currently authorized as a primary series only, and not as a booster dose.
Regulators authorized the vaccine following an extensive review of clinical trials and safety and effectiveness data. At the time of the announcement, the CDC stated that the Novavax vaccine would be available in the coming weeks.
Updated July 20, 2022
How does Novavax differ from the other available vaccines in the United States?
The Novavax vaccine is created using more traditional protein-based technology for vaccine development, unlike the other vaccines currently available in the United States (the Pfizer and Moderna mRNA vaccines and viral-vector Johnson & Johnson vaccine).
The Novavax vaccine uses a combination of purified coronavirus spike proteins and an immune-boosting stimulant called an adjuvant (common in many vaccines) to strengthen the body’s immune response against COVID-19. Novavax has already been authorized in more than 40 countries and has been granted emergency authorization from the European Union and the World Health Organization.
Updated June 29, 2022
What are the known side effects of the Novavax vaccine?
Data from the Novavax clinical trial also show that Novavax is more than 90% effective at protecting against symptomatic COVID-19, and 100% effective against severe illness and death. Common side effects include soreness at the injection site, fatigue, muscle pain, and headaches.
In terms of serious adverse reactions to Novavax, data show there were six cases of myocarditis from a clinical trial of about 30,000 people, primarily among young men. The cases of myocarditis in the clinical trial were treatable, and all six individuals recovered well. The risk of developing myocarditis from COVID-19 remains higher than the risk of developing it from a COVID-19 vaccine, including Novavax.
Updated June 29, 2022
Photo by Bryce Richter / UW-Madison